A Multi-Centre, Observational Study of Clozapine for Young People with Treatment Resistant Psychosis in Real World Settings
Co-sponsors: King’s College London and South London & Maudsley NHS Foundation Trust
REC: 22/LO/0723
Chief Investigator: Professor James MacCabe, Professor of Epidemiology and Therapeutics, King’s College London
Funded by National Institute for Health Research

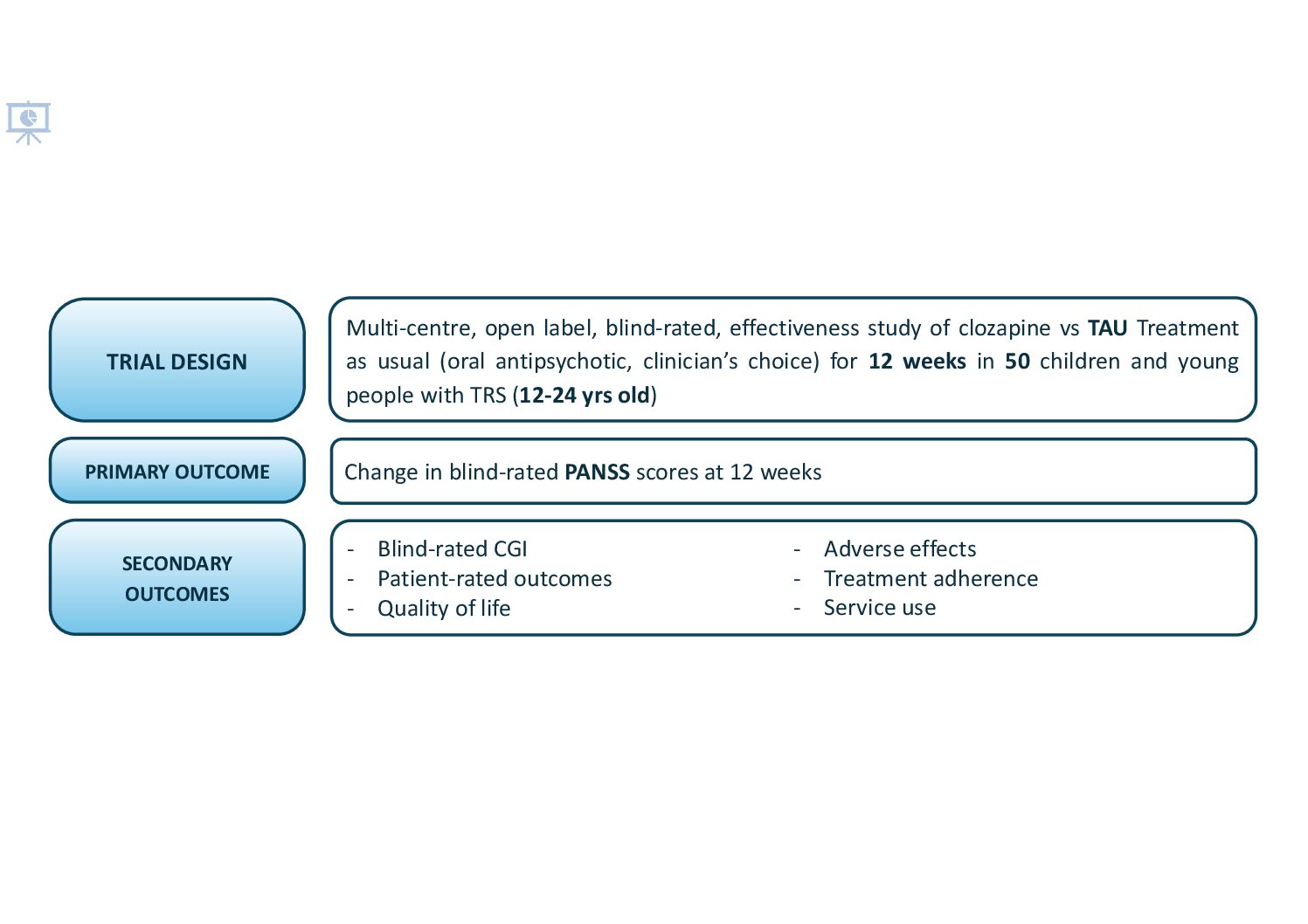
CLEAR (CLozapine in EARly Psychosis) is investigating the effectiveness of clozapine versus other antipsychotics (known here as treatment as usual or TAU) in young people under the age of 25.
Research on antipsychotics in young people and children shows that antipsychotics do help children and young people with psychosis. However, there is very limited research focussed on clozapine in young people, so it is not clear whether or not clozapine should be recommended for people under 25.
For these reasons, the National Institute of Health Research (NIHR) has commissioned this research to study clozapine as a treatment for psychosis in people under 25. The results will help the National Institute for Health and Care Excellence (NICE) decide which treatment they should recommend, so it should help other people to receive the best treatment in the future.
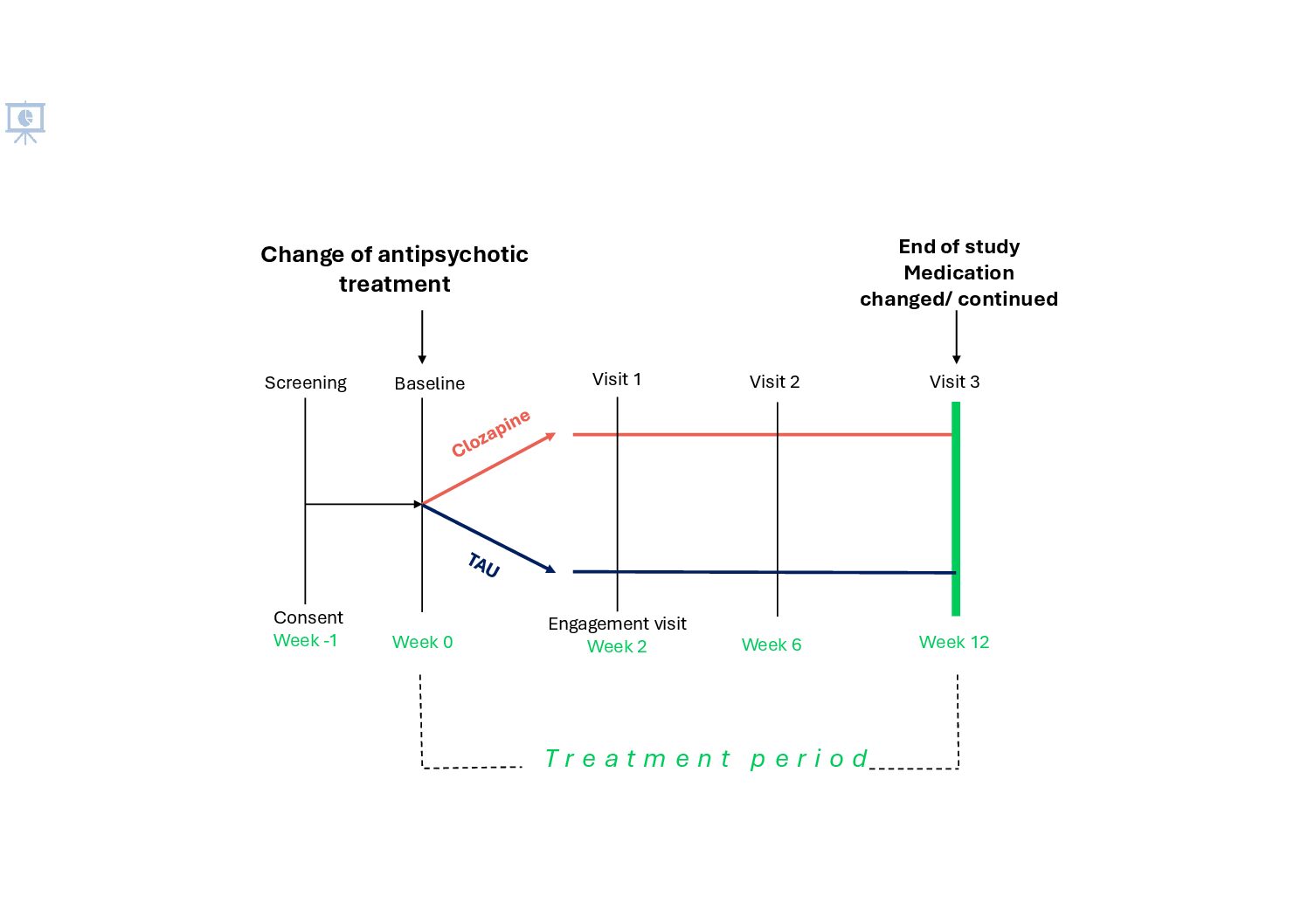
We plan to recruit 50 participants. Inclusion criteria includes young people with schizophrenia or a related disorder, aged between 12 to 24 years who meet NICE criteria for treatment resistance and are starting a new medication, having tried two antipsychotics previously. A researcher will meet the participant 4 times, firstly at a baseline visit prior to their new treatment commencing and then a further 3 times to complete some questionnaires and clinical assessments.
After 12 weeks of treatment, participants can discuss with their clinical team whether they want to continue the treatment or change to another medication.
Participants will be compensated for their time and travel expenses.
If you would like further information, please get in touch via email (clear@kcl.ac.uk)
The CLEAR study includes six participating academic centres, King’s College London, University College London, University of Oxford, University of Birmingham, University of Manchester and Cardiff University. These 6 hubs will act as a coordinating centre for their surrounding NHS Trusts and Health Boards acting as recruitment sites (approx. 25 across the UK, N.B. site list generated pre study start).
Co-applicants include Professor Paramala Santosh (South London and Maudsley NHS Foundation Trust), Professor Helen Killaspy (University College London), Professor Richard Emsley, Professor Sarah Byford, Professor Oliver Howes, Dr Cecilia Casetta (King’s College London), Dr Daniel Hayes (South London and Maudsley NHS Foundation Trust), Professor Richard Drake (The University of Manchester), Dr Rachel Elvins (Manchester University NHS Foundation Trust), Professor James Walters, Professor Jonathan Bisson (Cardiff University), Professor Belinda Lennox (University of Oxford), Dr Anthony James (Oxford Health NHS Foundation Trust), Professor Rachel Upthegrove (University of Birmingham), Dr Nicole Fung (Birmingham Women’s and Children’s NHS Foundation Trust), Dr Charles Dixon (Devon Partnership NHS Foundation Trust).


CLEAR is part of the NIHR Associate PI scheme
CLEAR is contributing to CONSULT SWAT, a study within a trial evaluating decision-making by families of adults who lack capacity to consent.