Supported Rescue packs post-discharge in chronic obstructive pulmonary disease: An open-label multicentre randomised controlled trial

Co-sponsors: King’s College London and Guy’s and St Thomas’ NHS Foundation Trust
REC: 24/SC/0186
ISRCTN: 44283921
Clinicaltrials.gov: NCT06347536
Chief Investigators:
Professor Mona Bafadhel King’s Centre for Lung Health, King’s College London
Professor John Hurst, UCL Respiratory, University College London

Chronic obstructive pulmonary disease (COPD) is a common lung condition in the United Kingdom, with a prevalence of 4.5% in population ≥40 years and rising4. In addition to daily symptoms such as cough and breathlessness that limit physical activity, people living with COPD are prone to unpredictable deteriorations in their health called ‘exacerbations’. Exacerbations are sometimes severe enough to lead to hospital admission and are often driven by infections. A systematic review of patient outcomes in COPD identified exacerbations, especially severe hospitalised exacerbations, as the aspect of COPD that patients found most difficult to live with5. Prior to the pandemic there were around 115,000 admissions to hospital with COPD exacerbations per annum6 and admissions are now returning to that level. Exacerbations are more common in the winter with greater circulation of respiratory viruses.Thus the burden of hospitalised exacerbations contributes to winter National Health Service (NHS) bed pressures and cost to the NHS. The annual healthcare cost for people with moderate and severe exacerbation of COPD in England was estimated to be nearly £1 billion in 20227. A particular problem after a hospitalised COPD exacerbation is re-admission to hospital. The National Asthma and COPD Audit Programme (NACAP) has shown that the re-admission rate is 23% at 30 days and 43% at 90 days2. Our systematic review identified comorbidities, previous exacerbations and increased length of stay as risk factors for 30- and 90-day all-cause readmission5.
There are many interventions that can reduce the risk of COPD exacerbations but these are not completely effective8. There is also evidence to suggest that earlier intervention with standard exacerbation treatment of antibiotics and/or corticosteroids (called a ‘rescue pack’) can hasten recovery, with a lessened chance of hospital admission9. As part of standard NHS care2, patients with COPD should have a ‘discharge bundle’ implemented, although this is often poorly delivered and has not been definitively shown to impact outcomes (likely because the wrong outcomes were chosen, and the bundle was poorly implemented)10. The provision of rescue packs is not a standard component of discharge bundles but these are sometimes provided according to local service preference3. Additionally, in usual clinical practice, some patients will have been prescribed rescue packs from primary care (GP) or a community respiratory team (CRT) prior to being hospitalised with COPD. Furthermore, patients may or may not have access to rescue packs from the GP or the CRT after hospital discharge.
Although rescue packs are part of NICE guidance2, the available evidence suggests they are not effective unless provided in the context of a more comprehensive management/education plan that supports patients in their appropriate use11. In practice this usually does not happen3, with evidence that a patient with COPD will receive variable or often no support. Some patients receive rescue packs on demand without considering antimicrobial resistance, predictable side-effects from steroid overuse, or reviewing appropriateness. The investigators have pilot data that show receiving a rescue pack on hospital discharge is controversial as the hospital team is not, in general, the team that provides ongoing support to use these. There is thus recognised over- and under-use of rescue packs, associated harm from these medicines and variable provision. Providing a rescue pack, with education on how to use and support for when to use, has not been specifically tested in the high-risk 90-day period for readmission following a hospitalised exacerbation.
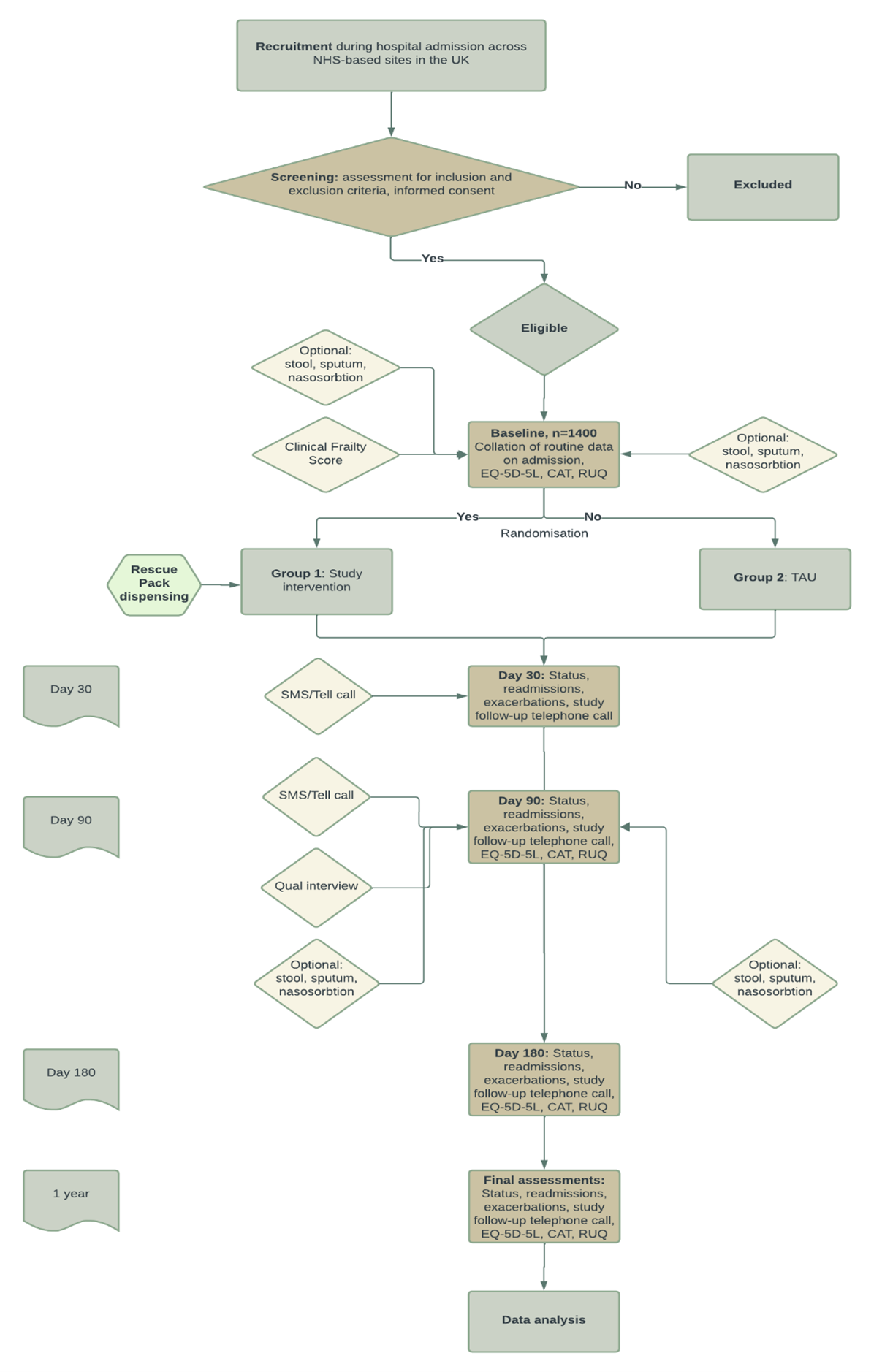
With RAPID (Supported Rescue packs post-discharge in chronic obstructive pulmonary disease: An open-label multicentre randomised controlled trial) we hypothesise that rescue packs on discharge in addition to a comprehensive self-supported management plan, consisting of the Asthma+Lung UK written management plan and twice weekly automated phone messaginge during 90 day high risk period, will reduce readmissions by 20% compared to standard care.
Aim
To test the hypothesis that provision of a rescue pack of antibiotics and corticosteroids, together with written (and translated) education on their use and twice-weekly telephone (or text) based support for when to use rescue packs, can reduce all-cause hospital re-admission in the 90-day high-risk period following discharge from hospital after an exacerbation of COPD.
Current protocol can be found here.
Inclusion Criteria:
- Adults aged 40 and over
- Patient to be discharged from hospital with exacerbation of COPD
- Able to provide informed consent
Exclusion Criteria:
- Requirement for invasive ventilation during the hospital admission
- Patients who have an expected survival of less than 90 days
- Patients with signs of new consolidation on chest X-ray (if available)
- Discharge to residential or nursing home
- Inability to engage with supported self-management
- No access to telephone
- Current participation in another interventional study
- Previous participation in the RAPID trial
King’s College London and Guy’s and St Thomas’ NHS Foundation Trust
University College London
Imperial College London
University of Cambridge
University of Bristol
University of Southampton
University of Nottingham
University of Leicester
Frimley Health NHS Foundation Trust
Newcastle University
Asthma+Lung UK
Sherwood Forest NHS Foundation Trust
Rotherham NHS Foundation Trust
North Tees and Hartlepool NHS Foundation Trust
Stockport NHS Foundation Trust
Blackpool Teaching Hospitals NHS Foundation Trust
Oxford University Hospitals NHS Foundation Trust
Somerset NHS Foundation Trust
University Hospitals Leicester NHS Trust
Mersey and West Lancashire Teaching Hospital NHS Trust
Barnsley Hospital NHS Foundation Trust
County Durham and Darlington NHS Foundation Trust
University of Sussex NHS Foundation Trust
East Suffolk and North Essex NHS Foundation Trust
South Tees NHS Foundation Trust
London North West University Healthcare NHS Trust
Chesterfield Royal Hospital NHS Foundation Trust
South Tyneside and Sunderland NHS Trust
King’s College Hospital NHS Foundation Trust
- https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1013597/life-sciences-vision-2021.pdf.
- https://www.nice.org.uk/guidance/NG115.
- Hurst JR, Quint JK, Stone RA, Silove Y, Youde J, Roberts CM. National clinical audit for hospitalised exacerbations of COPD. ERJ Open Res 2020;6.
- Trueman D, Woodcock F, Hancock E. Estimating the economic burden of respiratory illness in the UK. https://www.blf.org.uk/policy/economic-burden2016.
- Zhang Y, Morgan RL, Alonso-Coello P, et al. A systematic review of how patients value COPD outcomes. European Respiratory Journal 2018;52:1800222.
- Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care. National Clinical Guideline Centre 2004 Royal College of Physicians of London. http://www.nice.org.uk/nicemedia/live/13029/49425/49425.pdf.
- McLean S, Hoogendoorn M, Hoogenveen RT, et al. Projecting the COPD population and costs in England and Scotland: 2011 to 2030. Sci Rep 2016;6:31893.
- Agustí A, Calverley PM, Decramer M, Stockley RA, Wedzicha JA. Prevention of Exacerbations in Chronic Obstructive Pulmonary Disease: Knowns and Unknowns. Chronic Obstr Pulm Dis 2014;1:166-84.
- Wilkinson TM, Donaldson GC, Hurst JR, Seemungal TA, Wedzicha JA. Early therapy improves outcomes of exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004;169:1298-303.
- Hopkinson NS, Englebretsen C, Cooley N, et al. Designing and implementing a COPD discharge care bundle. Thorax 2012;67:90.
- Bailey WC, Richards JM, Brooks CM, Soong S-j, Windsor RA, Manzella BA. A Randomized Trial to Improve Self-Management Practices of Adults With Asthma. Archives of Internal Medicine 1990;150:1664-8.
CONNECT WITH US
King’s College London | Institute of Psychiatry, Psychology & Neuroscience | Psychosis Department | 16 De Crespigny Park | London SE5 8AF
rapid-rescue@kcl.ac.uk